
The Actinia® Hip Stem System features a complete range of cementless and cemented options designed for primary arthroplasty. In addition to standard stems the system offers stems with lateralised neck / offset geometry.
Indications:
- non-inflammatory degenerative joint diseases including osteoarthritis and avascular necrosis
- post-traumatic osteoarthritis
- fractures
- rheumatoid arthritis

Shoulder endoprosthetics
AGILON® Trauma prosthesis
The AGILON® modular shoulder system offers the surgeon great flexibility via an extensive range of modular components and has been designed for reconstruction following 3-fragment to 4-fragment fracture of the humeral head as well as arthritic scenarios. It is possible to change from the anatomic replacement option to the inverse option intraoperatively while leaving the bone anchoring components (i.e. the humeral stem and the glenoid) in place. With the help of extension pieces the proximal length of the implant can be extended from 5mm to 17.5 mm in steps of 2.5 mm.
The humeral caps feature eccentricity and come in a range of heights as well as a range of diameters which allows fine tuning of the medio-lateral offset.
Primary fixation of the cementless glenoid is augmented by locking screws. The inverse option utilises a polyethylene (PE) glenosphere and a thin metal humeral cap which reduces the risk of scapula impingement and hence increases the available range of motion. Additionally cuff tear arthropathy (CTA) caps are also available in a range of diameters and heights. Retentive inverse caps as well as cemented PE glenoids complete the system.

Product details
Indication:
Tumours in the area of the knee joint. Arthrodesis of the knee joint.
System components:
- Femoral stem cementless or cemented
- Arthrodesis
- tibial plate
- tibial stem cementless or cemented
Reconstruction length:
≥ 145 mm

The titanium alloy (TiAl6V4) MUTARS® LUMiC® Cup can be fixed within the wing of the ilium with either a modular cementless hydroxyapatite (HA) coated TiAl6V4 stem or a modular cemented matt-finished cobalt chromium molybdenum alloy (CoCrMo) stem. Once the stem of choice is implanted the orientation of the cup can be dialed in to optimise range of motion and bolted securely in place.
A range of insert options are available:
- 36mm internal diameter crosslinked ultra-high molecular weight polyethylene (UHMWPE) inserts with a 15° overhang to reduce dislocation risk – note also that a 4mm offset crosslinked UHMWPE liner is available which enables displacement of the center of rotation after definitive cup implantation.
- 42mm, 44mm or 46mm internal diameter 2M cobalt chromium molybdenum alloy (CoCrMo) ceramic-coated (TiNbN) 15° inlays which are highly stable in terms of managing potential dislocation
- 2M inlays (see details above) with corresponding implacross®E (vitamin E stabilised crosslinked UHMWPE) heads as a tripolar anti-dislocation option
Product details
Indications:
Partial pelvic reconstruction of the hip after complex revision or resection surgery – where there is a major acetabular defect
Materials:
cup, stems cementless: implatan®; TiAl6V4 according to ISO 5832-3
stems cemented, 2M inserts 15°: implavit®; CoCrMo according to ISO 5832-4
PE-inserts: implacross®; crosslinked UHMW-PE
heads: implacross® E; crosslinked UHMW-PE with vitamin E
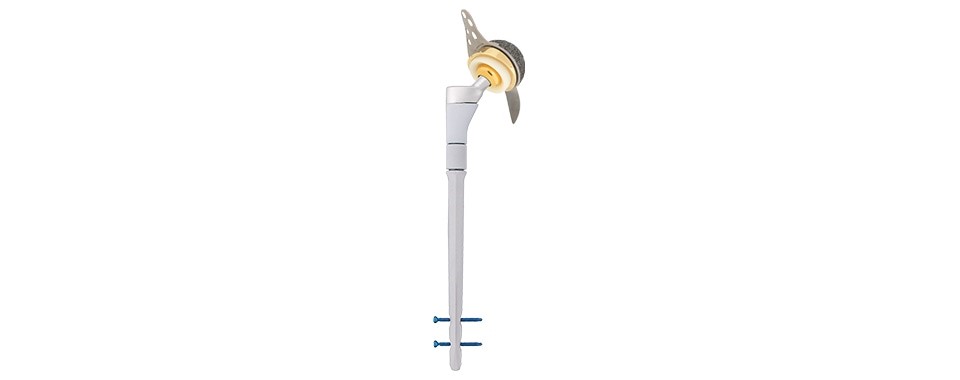
Proximal (metaphyseal) components
The MUTARS® RS system offers four titanium alloy (TiAl6V4) proximal component options each with a 12/14 morse taper
All of these hydroxyapatite (HA) coated cementless modular proximal options allow for the optimal choice and position of the actual distal diaphyseal stem to be defined first.
DIstal (diaphyseal) components
There are 31 hydroxyapatite (HA) coated titanium alloy (TiAl6V4) cementless stems available in the MUTARS® RS system in 3 different lengths (i.e. 150mm, 200mm and 250 mm) which come in 1mm increments:
- the 150mm length implants have diameters of 12mm to 22mm
- the 200 mm length implants have diameters of 12mm to 22mm (and the 15mm through 22mm sizes have a pair of distal locking holes)
- the 250 mm length implants have diameters of 14mm to 22mm (and the 17mm through 22mm sizes have a pair of distal locking holes)
The 12 matt-finished cobalt chromium molybdenum (CoCrMo) cemented stems in the MUTARS® RS come in 3 lengths (i.e. 120mm, 150mm and 200mm) and 4 different diameters (i.e. 12mm, 14mm, 16mm and 18mm).
Product details
Indications:
revision of a failed THR
Materials:
cementless: implatan®; TiAl6V4 according to ISO 5832-3
cemented: implavit®; CoCrMo according to ISO 5832-4

Product details
Indication:
Basic, cemented revision treatment of the knee without major bone defects.
Materials:
implavit®; CoCrMo according to ISO 5832-4
UHMW-PE according to ISO 5834-2
System components:
- GenuX®MK Monoblock femoral component cemented
- GenuX®MK Tibial component Monoblock cemented
- GenuX®MK PE-Insert
- MUTARS®GenuX® MK coupling
Stem length:
≥ 125mm tibial and femoral

Product details
Indication:
Tumours in the area of the distal femur.
System components:
- Femoral stem cemented or cementless
- Distal Femur M-O-M
- GenuX®MK PE insert
- MUTARS®GenuX® MK coupling
- GenuX®MK stem cemented or cementless
- GenuX®MK Tibial component cementless and cemented
- cemented Patella replacement
- GenuX®MK Offset adapter
- extension pieces
- connecting part
Reconstruction length:
≥ 100 mm

Product details
Indication:
Combined revision joint replacement of the hip and knee joint using an intramedullary connection with loss of distal femoral condyles.
System components:
- RS proximal component
- RS metaphyseal component
- RS Extension piece 25mm
- Intramedullary connecting module KRI
- KRI M-O-M
- MUTARS®GenuX® MK coupling
- GenuX®MK Tibial component cementless and cemented
- GenuX®MK Offset adapter
- GenuX® MK stem cementless and cemented
Reconstruction length:
≥ 327 mm (femoral length)

Concept
The TARIC® ankle system is designed as a cementless “Mobile Bearing“ system. Two fins are configured for the primary fixation of the tibial and talar component. These fins are configured so that they clamp the host bone. The additional applied cpTi- and HA-coating allows a solid secondary fixation of the metal components to the bone.
Articulating components
The system includes 5 sizes of tibial implants* and 4 sizes of talar components*. The highly congruent PE-inlays are made of UHMWPE (ultra high molecular weight polyethylene).

Product details
Indication:
Tumours in the area of the distal humerus
System components:
- Distal Humerus 50mm or 30mm cementless and cemented
- Ulna anchorage: cementless and cemented
- Humerus extension piece
Reconstruction length:
From 30mm for short component and from 60mm with Distal Humerus component

In order to minimise the micro movement that can cause polyethylene (PE) wear at the metal shell – PE liner interface, a special locking mechanism has been developed. This allows the use of a BIOLOX® delta ceramic insert or a PE insert with the same cup design. Alternatively PE inserts made of implacross® crosslinked polyethylene are available which demonstrated improved wear characteristics during preclinical tests.
EcoFit® cups have a central hole, in the acetabular base, for attaching the introducer for secure and controlled implant impaction. Once the cup is seated this central hole can be covered with a central screw plug. The EcoFit® cup also has three pre-plugged holes which can be removed, even when the cup is in-situ, for the insertion of supplementary cancellous screws to enhance primary stability (if required).
A 32mm articulation is possible with a 46mm diameter cup and a 36mm articulation is possible with a 50mm cup. This applies to implacross® and to BIOLOX® delta liners.
In addition to polyethylene and ceramic inserts the EcoFit® insert consisting of CoCrMo is also available.
Product details
Indications:
- non-inflammatory degenerative joint diseases including osteoarthritis and avascular necrosis
- post-traumatic osteoarthritis
- femoral neck fractures
- rheumatoid arthritis
Product range:
12 sizes (46-68mm)

The hemispherical ic-reinforcement cage is made of commercially pure titanium (cpTi). Initial primary stability is gained via a wedge-shaped ischial flange and a semi-circular illiac plate. Both of these implant features can be contoured to fit the available bony anatomy before being secured in place with flat-headed 6.5mm cancellous screws. The cup itself also has holes for cancellous screw augmentation/fixation to the available bone. In addition to this the bone-facing side of the implant is rough-blasted for enhanced fixation. The ic-reinforcement cage is available in four left and four right sizes. Once implanted the appropriate size of PE-Cup Mueller II or the appropriate size of EcoFit® 2M Cup should be cemented into place.
Product details
Indications:
- revision arthroplasty
- rheumatoid arthritis
- femoral neck fractures
- post-traumatic osteoarthritis
- non-inflammatory degenerative joint diseases including osteoarthritis and avascular necrosis
Materials:
pure titanium (cpTi) according to ISO 5832-2
Product range:
ic-reinforcement cage: 4 right-sided sizes and four left-sided sizes (44mm to 62mm in 6mm increments)
ic acetabular ring: 9 sizes (42mm to 58mm in 2mm increments)

The ic-head titanium options are made of titanium alloy (TiAl6V4) and are coated with titanium nitride (TiN) to make them suitable for use as a load bearing articulating surface. The ceramic coating process also makes these devices especially useful for the Treatment of patients with metal sensitivity.
ic-head CoCrMo which are made of cobalt chromium molybdenum alloy.
Product details
Coating:
ic-head titanium: TiN-coating
Materials:
ic-head titanium: implatan®; TiAl6V4 according to ISO 5832-3
ic-head CoCrMo: implavit®; CoCrMo according to ISO 5832-12
Note: The combination of ic-heads XXL and XXXL is restricted.
Product range:
ic-head titanium: 28mm (K-XXXL), 32mm (K-XXXL), 36mm (K-XXXL)
ic-head CoCrMo: 22mm (K-L), 28mm (K-XXXL), 32mm (K-XXXL), 36mm (K-XXXL)

